Introduction. In response to a growing and increasingly older population with complex medical needs, the hematology and oncology (HO) care system has integrated the hospitalist model into inpatient systems alongside HO trainees and faculty. The inclusive workforce is comprised of hospitalists and advanced practice providers (APPs) who co-manage patients with specialists or form separate non-trainee (NT) teams. Despite multiple advantages of the hospitalist model, the optimal care team structure and the impact on HO fellowship training have not been previously explored. We aimed to survey HO fellowship programs in the United States to identify the structure of inpatient HO staffing models implemented at their institution, and collect data on perceptions of the NT model within the fellowship programs.
Methods. We constructed and distributed two national web-based surveys targeting all adult HO fellowship program directors (PDs) and fellows from Accreditation Council for Graduate Medical Education (ACGME)-accredited programs. The surveys were designed to assess various aspects of HO inpatient services, including team compositions, individual responsibilities, and availability of NT services. We also assessed the PDs' and fellows' perception on the impact of NT teams on their training and education. The surveys were emailed weekly between 6/20/23-7/29/23.
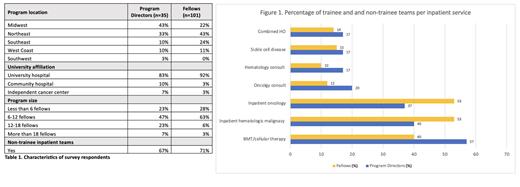
Results.We identified 147 HO training programs.A total of 35 PDs and 101 fellows responded our survey (Table 1). The majority of PDs (83%) were from university training programs in the Midwest (43%) and Northeast (33%), with fellow class sizes of 6-12 (47%). Most (67%) programs had both trainee (T) and NT teams included in their inpatient medicine models (Figure 1). The most commonly reported NT team was the bone marrow transplant and cellular therapy service (BMT) (57%), supported primarily by APPs (55%). Only 33% of respondents reported a dedicated sickle cell disease (SCD) inpatient team within their institution. Hospitalists were included in the care model of the inpatient oncology (27%), inpatient malignant hematology (23%) and SCD (13%) teams. Most PDs agreed that the fellows are primarily responsible for most inpatient tasks and over 50% agreed that APPs are responsible for direct patient care, admissions and discharge planning. 83% of PDs reported that their program had an appropriate balance between inpatient and outpatient care. Half (50%) of PDs reported that fellows had a positive perception of NT teams at their institution, and 35% were neutral. The fellows who responded were predominantly second-year fellows (43%) in Northeastern (43%) university hospitals (92%) from programs with 6-12 fellows (63%). Most (71%) respondents had both T and NT teams included in their inpatient model. Inpatient malignant hematology (53%) and inpatient solid oncology (53%) teams were the most commonly reported NT teams, followed by BMT (40%) (Figure 1). Fellows reported their primary responsibilities to include procedures and tumor board discussions (94%), communication with outpatient teams (91%) and resident education and supervision (89%). APPs were primarily responsible for discharge planning (63%), admissions (59%) and direct patient care (58%). Most (85%) fellows agreed that their program had an appropriate balance between inpatient and outpatient care. Most fellows (69%) reported the impact of NT teams on fellows' inpatient and training experience to be neutral. Fellows from institutions without NT teams responded that the addition on NT team would have a positive impact in their training experience (69%). Most PDs and fellow respondents perceived a financial barrier to the implementation of NT teams at their institutions.
Conclusions.Our findings highlight the existing heterogeneity in the distribution and composition of services among HO programs nationwide. PD respondents and fellows without NT teams perceived these teams as a beneficial addition to the program, in contrast to fellows in programs with existing NT teams who reported a neutral impact. Further investigation of the reasons for this discrepancy is essential to identify areas of improvement. Additional insights from the ongoing survey will be presented during the meeting.
Disclosures
Stahl:Haymarket Media: Other: GME activity ; Curis Oncology: Other: GME activity ; Boston Consulting: Consultancy; Dedham group: Consultancy; Clinical care options: Other: GME activity ; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: GME activity ; Sierra Oncology: Membership on an entity's Board of Directors or advisory committees; Rigel: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Kymera: Membership on an entity's Board of Directors or advisory committees. Podoltsev:Cogent Biosciences: Other: IDMC Member; AI Therapeutics; Arog Pharmaceuticals; Astellas Pharma, Inc.; Astex Pharmaceuticals; Boehringer Ingelheim Pharmaceuticals, Inc.; Celgene Corporation; CTI BioPharma Corp.; Daiichi Sankyo, Inc.; Genentech, Inc.; Jazz Pharmaceuticals, Inc.; Kartos Therapeuti: Research Funding; AbbVie Inc.; Blueprint Medicines (former); Constellation Pharmaceuticals (former); CTI BioPharma Corp. (former); Incyte Corporation (former); Novartis (former); PharmaEssentia (former): Consultancy. Shallis:Bristol Myers Squibb: Consultancy; Curio Science: Consultancy; Rigel: Consultancy; Servier: Consultancy; Gilead Sciences: Consultancy. Rangachari:Advance Medica/TelaDoc: Honoraria; DynaMed: Honoraria; Bristol Myers Squibb: Research Funding; DAVA Oncology: Other: Travel support; Novocure: Research Funding; AstraZeneca: Honoraria; AbbVie/Stemcentrx: Research Funding. Michaelis:NKARTA: Consultancy; Sierra oncology: Consultancy; Celgene Corporation: Consultancy; Sierra Oncology: Consultancy; Jazz Pharmaceuticals: Consultancy, Research Funding; Incyte Corporation: Consultancy. Zeidan:Boehringer-Ingelheim: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Shattuck Labs: Research Funding; Seattle Genetics: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Astex: Research Funding; Kura: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Foran: Consultancy, Research Funding; Otsuka: Consultancy, Honoraria; Geron: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Notable: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Ionis: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria.